For the past year, the world has been consumed with anxiety surrounding the coronavirus pandemic. Now, with multiple vaccines authorized and being administered in various countries, a light is beginning to appear at the end of a very dark tunnel.
The coronavirus vaccines were developed at record-breaking speed. Typically, the drug development process can take up to a decade involving research, testing and clinical trials. And to help companies through this process by creating touchpoints for research, education and marketing are agencies such as BCD Meetings & Events, specializing in life sciences meetings management. In this article, we detail the various meeting types involved in the drug development process.
Meetings involved in the the drug development process
There are four stages of the drug approval process, from discovery and development to FDA review. Once a medicine is approved, a fifth stage takes place to market the product.
Throughout its lifecycle, different types of meetings take place depending on what stage the product is at. Each goes through a similar process in its clinical development and marketing. Throughout each phase, meeting types can change depending on the objective.
These meetings help Life Sciences companies gain expert input from doctors within the industry and train medical professionals on clinical trials. Once a product is approved, internal meetings are hosted to train sales team and officially launch the product.
At BCD M&E, we proudly service 90% of the world’s top pharmaceutical companies within our Life Sciences Center of Excellence, which specializes in these meeting types. To better understand the role meetings play in the drug approval process, here is further explanation of the meetings involved.
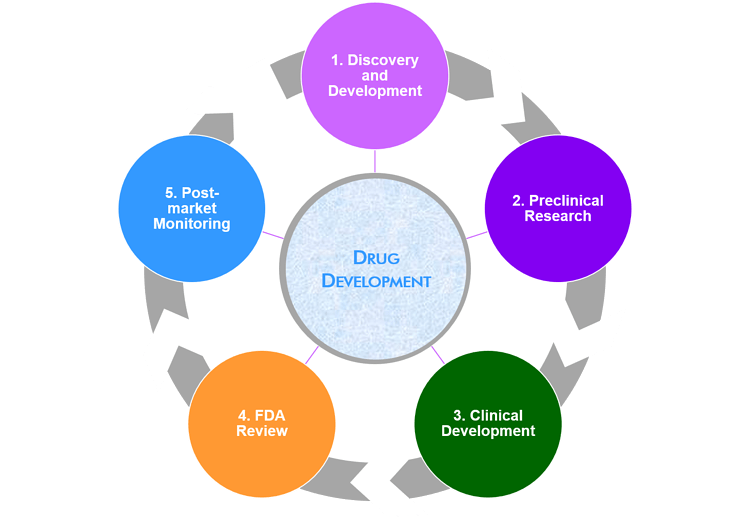
Phase 1: Discovery and Development
During discovery, researchers conduct early testing of molecular compounds for potential candidates for development. Once a probable compound has been identified, experiments are conducted to collect information on the benefits, dosage and side effects.
Clinical Trial Meeting
The goal of this type of meeting is to train healthcare professionals on how to administer the drug and what data to gather during the clinical trial.
Advisory Boards
These meetings connect the pharmaceutical company with industry experts to seek advice and discuss data to better understand a therapy area.
Phase 2: Preclinical Research
In the preclinical phase, researchers analyze the safety and efficiency of a product, with the goal to decide whether it is safe to test in humans.
Investigator Meeting
These meetings can vary in purpose but always with the intention of training and keeping sites (where a clinical trial or study is conducted) engaged and patients enrolled. They train sites on the study protocol and often offer refresh trainings or updates as they move through the phases.
Clinical Research Associates Training
Study teams will also use investigator meetings as an opportunity to host CRA trainings. CRAs (or trial monitors) oversee clinical trials on behalf of pharmaceutical companies. They are “assigned” to certain sites and networking with them at meetings is important.
Also involved in this phase:
-
- Advisory Boards
- Clinical Trial Meeting
Phase 3: Clinical Research & Development
Clinical research studies how a drug will interact with the human body and includes research that is done in people.
Sponsored Symposia
These are scientific lectures performed by an expert speaker at a congress organized by a medical association and attended by HCPs interested in the topic.
Also involved in this phase:
-
- Advisory Boards
- Investigator Meetings
- KOL Meetings
Phase 4: FDA Drug Review
Once a drug developer collects evidence from its research that a drug is safe and effective, the company can file an application with the FDA (or EMA in Europe) to market the drug.
Product Launch
The goal of this meeting is to introduce the product once it has been approved to sell. Generally, this is an internal launch within the company to include all colleagues working on the specific product.
Product Training
Attended by internal and cross-functional teams, the purpose of this meeting is to train the attendees on the product.
Also involved in this phase:
-
- Advisory Boards
Hosting in a virtual format
Since the onset of the coronavirus outbreak, our teams have been able to successfully transition these meetings to virtual formats. Due to the pandemic, most HCPs involved are also supporting the fight against COVID-19, so the convenience of virtual meetings has made it easier for them to attend. This has also contributed to a wider group of participants. One factor that has been a challenge is creating sufficient networking opportunities within a virtual format, which is a major benefit for HCPs attending these types of meetings.
These meeting types are vital in the drug approval process. The patients involved and data collected and reported on is crucial. The communication between various functional teams within the Life Science company and externally with KOLs (key opinion leaders) is fundamental to the drug development process and meetings play a key role in bringing stakeholders together.
Originally published Mar 8, 2021 10:00:00 AM
Last updated on Jan 3, 2023 11:06:10 AM


